40 Cell cycle regulation
Cell growth and division are moments within a larger process known as the cell cycle. The cell cycle impacts every aspect of cellular function. Every organelle gets involved in some way or another at every step of the way. Growth requires protein and membrane synthesis, energy production, transport of materials, and more. Selecting the correct moment to undergo cell division requires careful coordination of both the internal and external environment of the cell. Cells that divide at the wrong moment are unlikely to survive the process, so it must be carefully timed and controlled.
Dive deeper
Watch this video explaining the cell cycle and mitosis: Neural Academy. (2019, August 6). Mitosis, Cytokinesis, and the cell cycle [YouTube, 8:34mins]
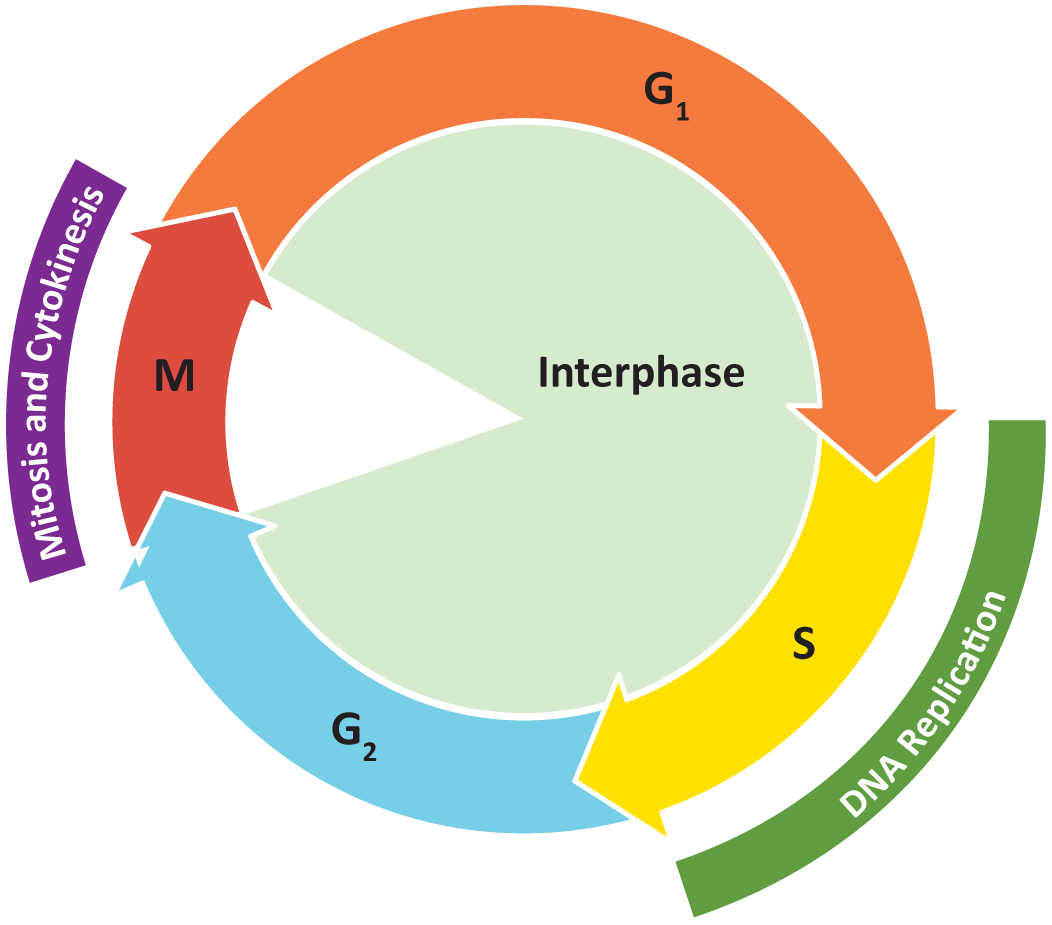
The cell cycle is the events that enable cells to proceed from one cell division event to the next. Cell division itself consists of the overlapping processes of mitosis (nuclear division) and cytokinesis (division of the cytoplasm).
Phases of the cell cycle
- G1 (gap or growth 1) phase This is between the end of cytokinesis and the start of DNA synthesis. It involves cell growth.
- S (synthesis) phase DNA synthesis occurs.
- G2 (gap or growth 2) phase From the end of DNA synthesis to the onset of mitosis. The cell continues to grow and prepare.
- M (mitosis) phase Cell division occurs.
Collectively, we consider G1, S, and G2 to be interphase (Figure 8.12).
Signalling and regulation are at the heart of the proper progression of the cell cycle. Using these tools, the cell ensures that:
- DNA synthesis and mitosis, do not occur before the cell is ready
- the cell pauses DNA replication or mitosis when errors are identified and repairs are attempted
- some cells stall temporarily, or even go through programmed cell death (apoptosis)
- growth is coordinated with cell division so that the size of cells is maintained over the many cellular generations
One of the most important controls placed on the progression of the cell cycle is a series of checkpoints in which the cell is required to meet certain criteria before it is allowed to proceed. These checkpoints act as a form of quality control. Each of these checkpoints is controlled by one or more ‘gatekeeper proteins’ that respond to cellular conditions and will only allow the cell to move forward into the next phase of the cell cycle if conditions are right.
- G1/S checkpoint allows the cell to pass into S phase
- G2/M checkpoint controls when the cell enters mitosis
- Metaphase checkpoint is in the middle of mitosis and ensures that the mitotic spindle is set up correctly prior to chromosome separation
Activated cyclin-CDK complex
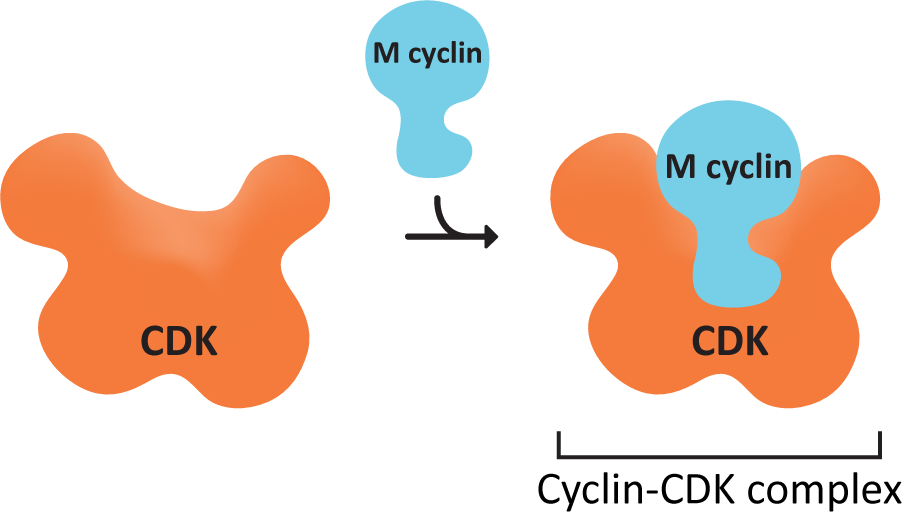
A set of proteins control the checkpoints and so control the entire cell cycle. The active agent is a protein called cyclin-dependent kinase (CDK).
A kinase is an enzyme that specifically adds phosphate groups to other proteins.
This particular kinase is only active in the presence of a second protein, cyclin, which binds to CDK (Figure 8.13), and acts as a regulatory unit. If cyclin is removed from CDK, then CDK is inactivated. These two proteins combine to produce a single enzyme called the cyclin-CDK-complex. The cell controls the activity of CDKs by controlling the synthesis and destruction of cyclins.
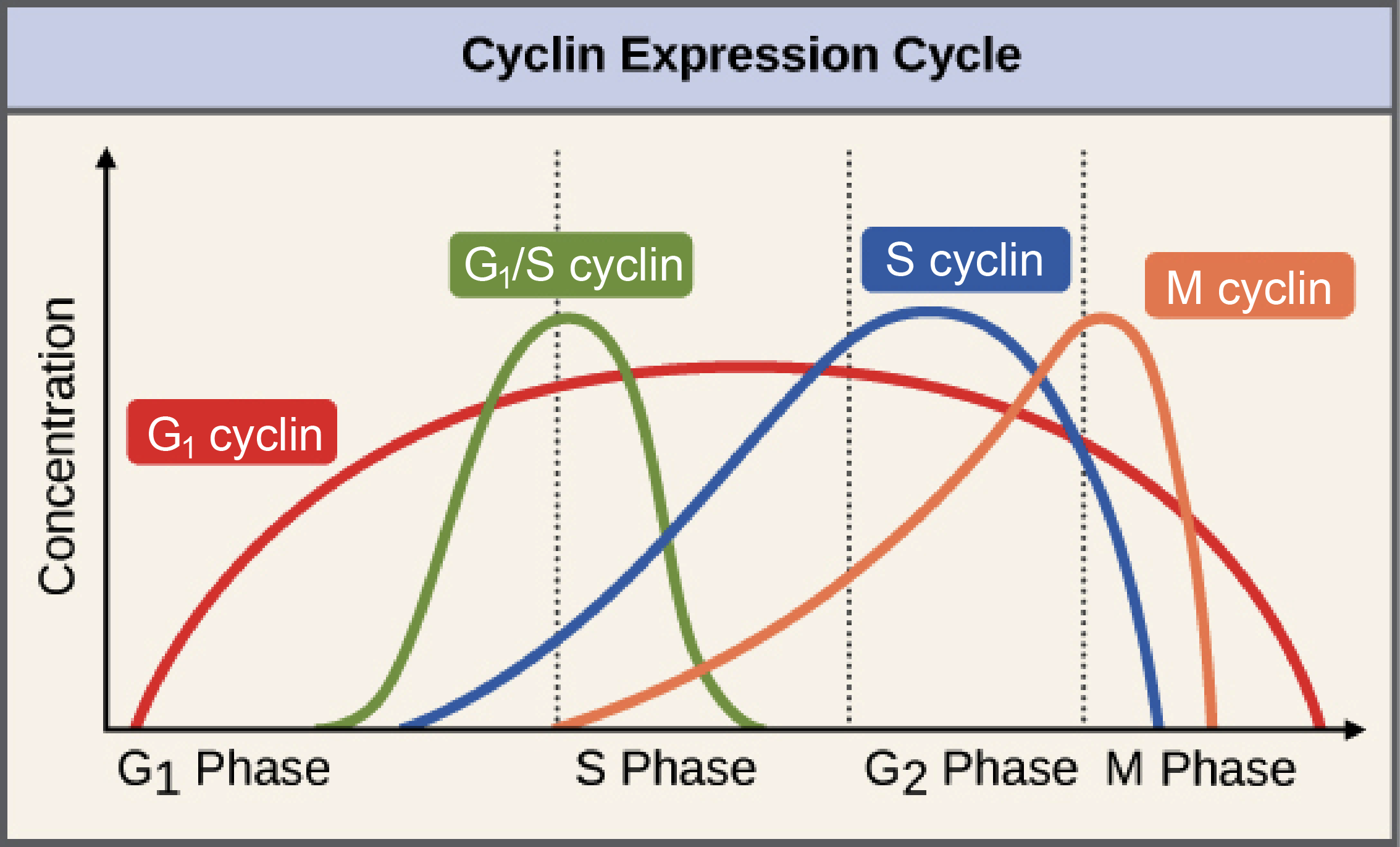
CDK concentrations in the cell remain constant throughout the cell cycle, but they are not always active. Cyclin concentrations increase as the cell moves through the cell cycle, which coincides with increasing enzymatic activity of CDK, peaking at the appropriate point in the cell cycle (usually a checkpoint). After the relevant checkpoint is passed, cyclin concentrations crash down to almost nothing. It is this “cycling” of the cyclin concentrations that regulates CDK activity, allowing the cell to progress through checkpoints of the cell cycle. There are several classes of cyclins and CDKs, and each of these classes is responsible for controlling a specific part of the cell cycle.
Increasing cyclin concentrations occur directly prior to a cell passing a cell cycle checkpoint because the cyclin-CDK activity must hit a certain level before the checkpoint can be passed, and cyclins are required components of CDK activity. In some cases, such as the M cyclin (Figure 8.14), the activity of the CDK decreases rapidly not long after the checkpoint has been passed, but in others, like the G1-cyclin shown here, the activity of the CDK is activated at one checkpoint and then stays high throughout the rest of the cycle.
Phosphorylation controls cyclin-cdk enzymatic activity. The activity of the CDKs must be very tightly controlled. due to the role they play in cell proliferation and programmed cell death (aopotosis).
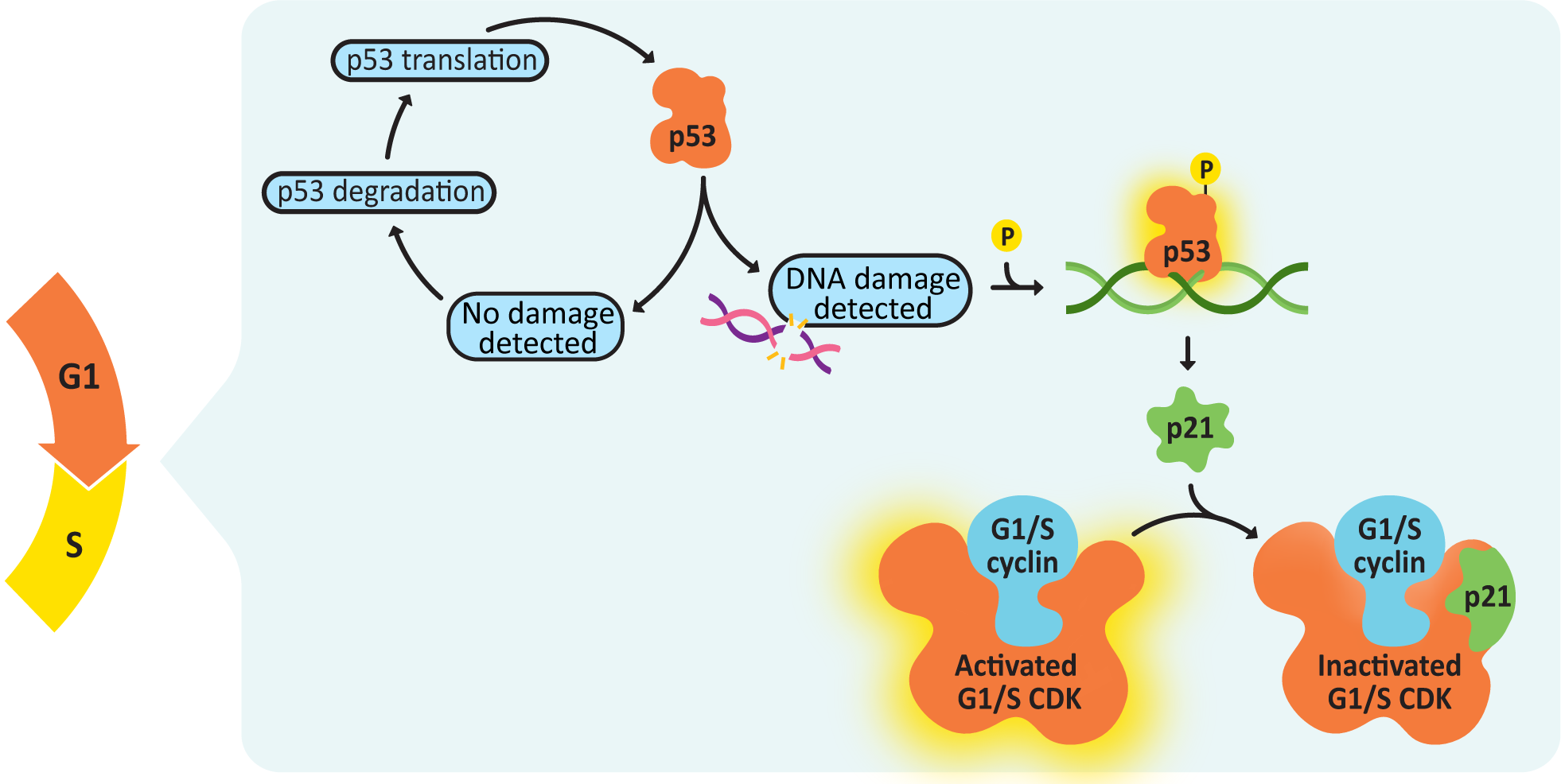
The transition from G1 to S phase is controlled by multiple cyclin-CDK complexes, including a G1 cyclin-, an S-cyclin-, and a G1/S cyclin-CDK complex (Figure 8.14). The G1/S cyclin-CDK complex will be de-activated once the checkpoint is passed, but the others will continue to function. Once the G1/S checkpoint is passed, DNA replication will begin.One of the most important criteria for the passing of the G1/S checkpoint is that there can be no detectable DNA damage. The protein that manages checking for DNA damage is a transcription regulator, p53. In its inactive state, p53 is constantly translated and then immediately degraded (Figure 8.15). When DNA damage is detected, p53 gets phosphorylated, which stops it from being degraded. It then binds to the promoter sequence for a CDK inhibitor, p21, activating its transcription and subsequent translation. p21 blocks the activity of the G1/S cyclin-CDK complex, which will stop the cell from passing the G1/S checkpoint.
 Case study
Case study
Nala, a 10-year-old intact female Domestic Shorthair cat, was presented to the veterinary clinic with a firm, irregular mass in the left caudal mammary gland. The owner reported that the mass had grown noticeably over the past month and that Luna had become more withdrawn and less active. On physical examination, the mass was approximately 3 cm in diameter, fixed to underlying tissues, and associated with mild regional lymphadenopathy. No other abnormalities were noted on general examination.
A comprehensive diagnostic plan was initiated to characterise the mass and assess for metastasis. Fine needle aspiration suggested a malignant epithelial neoplasm, prompting surgical biopsy and histopathological evaluation. Thoracic radiographs and abdominal ultrasound were performed to check for metastatic spread, which revealed no distant metastases at the time of diagnosis. Histopathology confirmed a diagnosis of mammary adenocarcinoma, a common and aggressive tumour in intact female cats. Immunohistochemical (IHC) staining revealed over-expression of mutant p53 protein, indicating a mutation in the TP53 tumor suppressor gene.

 Reflective question
Reflective question
Is the fact that the cat was not spayed relevant to the case study above?
p53 is mutated in as many as 50% of all cancers, which is a clear indication of just how important this protein is in the proper function of the cell cycle. If you lose p53 function in a cell, you lose your ability to delay the cell cycle so that there’s time to repair DNA damage. At that point, damage is less likely to get fixed before replication, and mutations accumulate at a much faster rate, with each new round of replication.
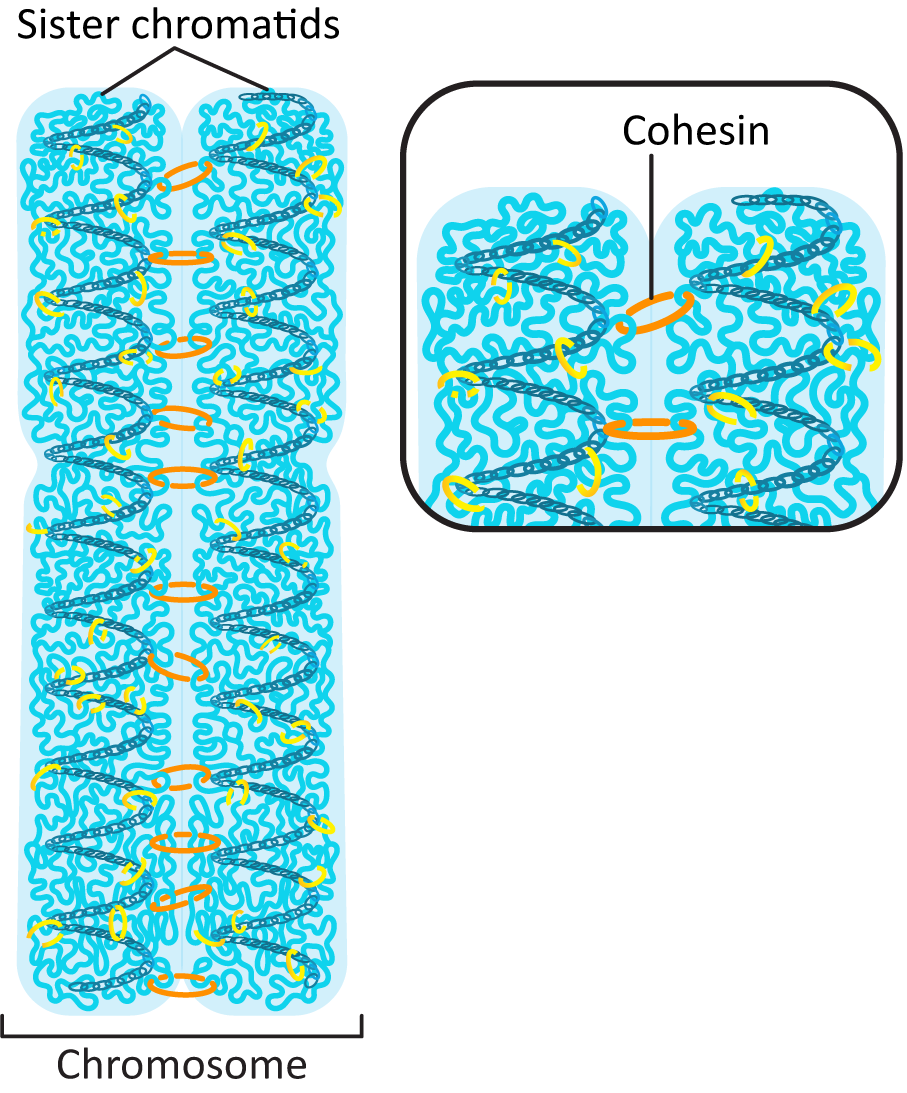
Prior to the start of S phase, each chromosome in the genome was made of a single helix of DNA that is in a complex with histones to form a chromatin fibre. It has its own telomeres at each end of the chromosome and a centromere near the middle of the DNA. During DNA replication, the DNA double helix is duplicated, and the chromosome now includes two sister chromatids held together by a protein, cohesin, until they part during mitosis (Figure 8.16).
Deactivation of the cyclin-CDK complex
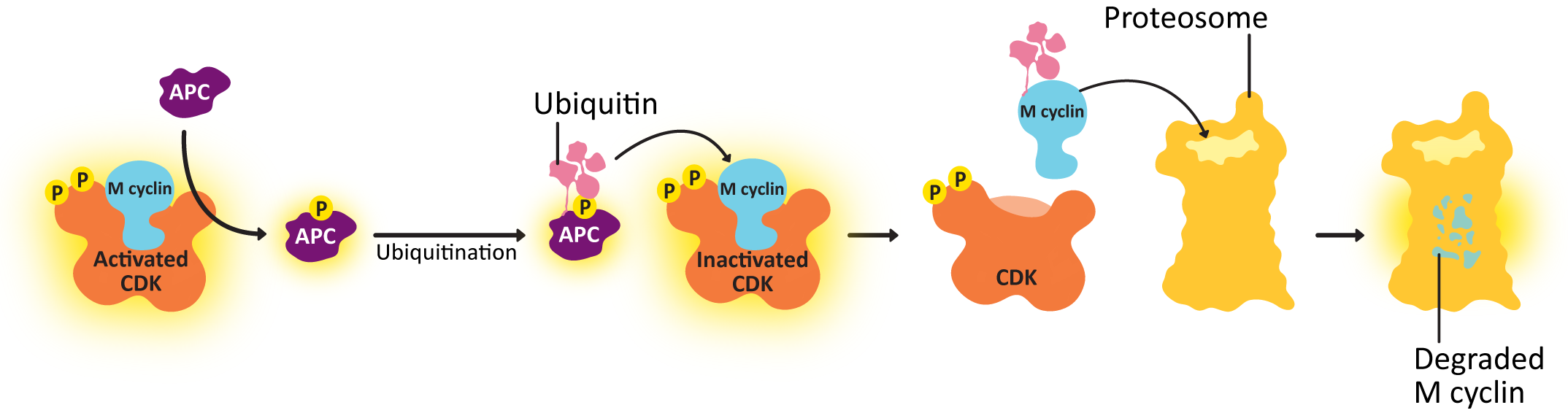
To end M phase, M cyclin-CDK must be deactivated and stop phosphorylating its target proteins. Deactivation of the cyclin-CDK complex involves:
- shutting down the transcription and translation of new cyclin and
- degrading the cyclin proteins that already exist
Anaphase-promoting complex (APC) is a protein that transfers a small protein tag called ubiquitin to other proteins, and it tags the cyclin with ubiquitin, which results in it being sent to the proteasome for degradation. Once the cyclin is degraded, the CDK is no longer active, and all of the phosphorylation targets of the CDK can then be dephosphorylated and returned to their original state. The result is that the concentration of cyclin in the cell drops rapidly, and the CDK is also deactivated, and the cell can start the process of putting the cytosolic contents back together again in the new daughter cells, complete cytokinesis, and return to G1. For its part, APC is deactivated in G1. It is one of the phosphorylation targets of the activated G1/S-cyclin-CDK complex.