25 Carbohydrates
What are carbohydrates?
Carbohydrates are the major components of plant tissue, making up to 60% to 90% of the dry matter. Carbohydrates contain carbon, hydrogen, and oxygen in the proportion found in water (CH2O) and are hence hydrates of carbon. Carbohydrates are the basic energy source in animal cells. Dietary carbohydrates obtained from plant-based products serve as a major source of energy for the animal. The chlorophyll in plant cells traps solar energy and produces carbohydrates using carbon dioxide and water and gives off oxygen, as shown in the following equation:
energy + 6 CO2 + 6 H20 → C6H2O + 6 O2
Carbohydrates are the major dietary source of energy for animals
In the plant cell, carbohydrates could be present in the cell content as sugar or starch, or they could be associated with the cell wall structure (for example, cellulose). When animals eat plant materials (for example, cereal grains, grass, fodder), energy in the feed’s carbohydrates is made available through metabolic processes in the animal cell. Overall, animal metabolism produces energy in a reverse process to that of photosynthesis.
Animal metabolism produces energy in a reverse process to that of photosynthesis in plants.
Structure and classification of carbohydrates
One method of classifying carbohydrates is based on the number of carbon atoms per each molecule of a carbohydrate and on the number of molecules of sugar in the compound. Based on the number of carbon atoms, a carbohydrate can be classified as triose (3 C), tetrose (4 C), pentose (5 C), and hexose (6 C). The suffix “ose” at the end of a biochemical name flags the molecule as a “sugar.” Among these, pentoses (e.g., ribose in ribonucleic acid (RNA)) and hexoses (e.g., glucose, or blood sugar) are the most common sugars in animal tissues. Based on the number of molecules of sugar in the compound, carbohydrates can be classified as (1) monosaccharide, one unit of sugar; (2) disaccharide, two monosaccharides; (3) oligosaccharide, three to fifteen monosaccharides; and (4) polysaccharides, large polymers of simple sugars.
Monosaccharides are often referred to as simple sugars (e.g., glucose; Figure 5.6) and cannot be hydrolysed into simpler compounds.
Monosaccharides can be subdivided based on the number of carbon (C) atoms. The following list shows the prefixes for numbers of carbons in a sugar:
- Triose (3 C)
- Tetrose (4 C)
- Pentose (5 C; e.g., Xylose and Ribose)
- Hexose (6 C; e.g., glucose, fructose, galactose, and mannose)
Monosaccharides are the simplest forms of carbohydrate.
Most monosaccharides in animal tissues are of 5 C and 6 C sugars. Simple sugars are also subdivided into aldose, a sugar that contains an aldehyde structure, or ketose, a sugar that contains a ketone group. Both glucose and fructose have the same molecular formula C6H12O6 and are hexoses (6 C). But glucose is an aldose (also called aldohexose) and fructose is a ketose, or a ketohexose.
The three hexoses that are nutritionally and metabolically important are glucose, fructose, and galactose.
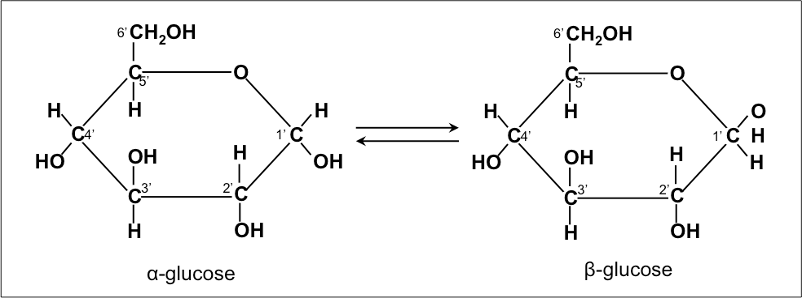
Most nutritionally important sugars are pentoses or hexoses.
The chemical structure of glucose can be represented as a straight chain form and in cyclic form. In a biological system, glucose exists primarily as a cyclic form and very rarely in a straight form (in aqueous solution). Glucose is the form of carbohydrates found in circulating blood (blood sugar) and is the primary carbohydrate used by the body for energy production. Fructose, or “fruit sugar,” is found in ripened fruits and honey and is also formed by digestion of disaccharide sucrose. Galactose is found along with disaccharide lactose in mammalian milk and is released during digestion.
Starch contains α-D-Glucose, while cellulose has rigid polymers with β-D-Glucose. Nutritionally important sugars are of the D-form (not the L-form).
Nutritional important sugars are of the D-form.
Disaccharides are made up of two monosaccharides bonded together by a glycosidic (covalent) bond. The following are some of the common disaccharides:
- Sucrose-glucose + fructose (e.g., table sugar)
- Lactose-glucose + galactose (milk sugar)
- Maltose-α-D-Glucose + β-D-Glucose (malt sugar)
- Cellobiose-β-D-Glucose + β-D-Glucose (cellulose)
Among the different disaccharides, lactose (milk sugar) is the only carbohydrate of animal origin. However, cellobiose as a component of cellulose is important in animal nutrition. Monogastric animals cannot digest cellulose because they do not produce the cellulase enzyme that can split β-D-Glucose.
Oligosaccharides are made by bonding together three or more (3 to 15) monosaccharides bonded together:
- Raffinose (glucose + fructose + galactose; 3 sugars)
- Stachyose (glucose + fructose + 2 galactose; 4 sugars)
In animal diets, oligosaccharides are commonly found in beans and legumes. Some oligosaccharides are used as substances to enhance the growth of good microbes (prebiotics). Recently, there has been an increased interest in the use of different oligosaccharides as feed additives to enhance hindgut health (e.g., fructooligosaccharides, mannan oligosaccharides).
Polysaccharides are made by joining together large polymers of simple sugars.
Polysaccharides are the most important carbohydrate in animal feed. Polysaccharides are composed of many single monosaccharide units linked together in long, complex chains. The functions of polysaccharides include energy storage in plant cells (e.g., seed starch in cereal grains) and animal cells (e.g., glycogen) or structural support (plant fibre). Components of cell wall structure are also called non-starch polysaccharides, or resistant starch, in animal nutrition, as they cannot be digested by animal enzymes but are fermented by hindgut and rumen microbes.
Polysaccharides can be homopolysaccharides or heteropolysaccharides.
Examples of homopolysaccharides that are important in animal nutrition include starch (nonstructural form), glycogen (animal form), and cellulose (plant structural form).
Starch is a principal sugar form of carbohydrate in cereal grains (seed energy storage). Starch is the chief carbohydrate source in the diet of monogastric animals. Forms of starch in cereal grains include:
- Amylose is the simplest of the polysaccharides, is water soluble and constitutes 15% to 30% of total starch in most plants.
- Amylopectin
Glycogen is a form of starch found in animal tissue and is hence called animal starch. Glycogen is a polysaccharide that exists in a small amount (< 1%) in liver and muscle tissue.
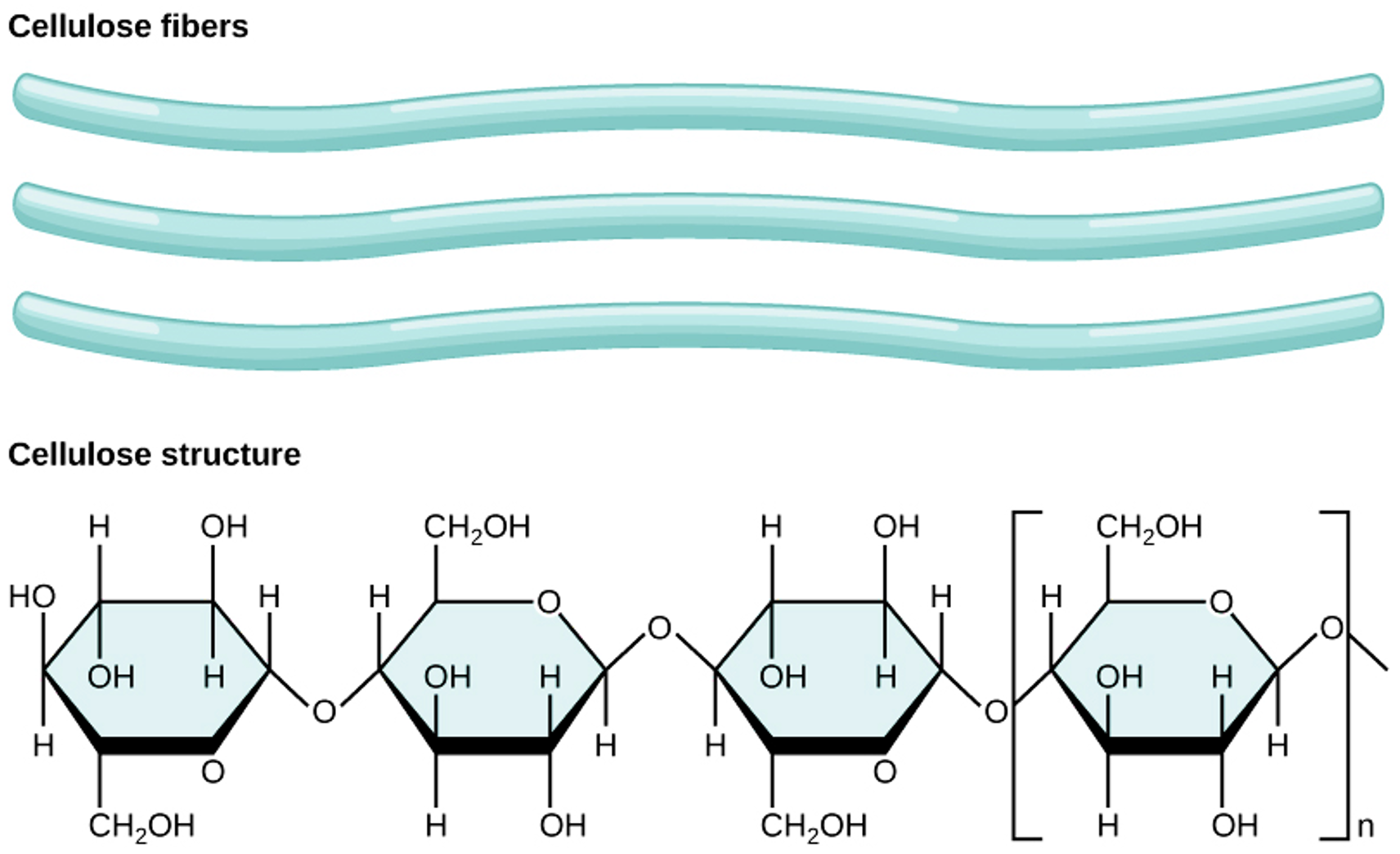
Cellulose is the most abundant carbohydrate in nature. It provides structural integrity to plant cell walls (Figure 5.7). Cellulose is highly stable. No animal enzyme can break it; only microbial cellulase can degrade it. Ruminant animals such as cattle, however, have bacteria in their rumen that contain the enzyme cellulase. It breaks the beta 1,4 links of the glucoses in cellulose to release the sugar for energy.
Dive deeper
Watch this video on carbohydrates: RicochetScience. (2015, November 4). Carbohydrates [YouTube, 4:24mins]
Metabolic pathways of glucose and fatty acids to produce energy in the animal body
Forms of energy
Hydrogen plays a prominent role in energy metabolism. During the catabolism of glucose (C6H12O6) by the animal, hydrogen is transferred from glucose to hydrogen receptors, such as nicotinamide adenine dinucleotide (NAD+) and flavin adenine dinucleotide (FAD). These hydrogen acceptors (reducing equivalents) are oxidized in the reactions of the respiratory chain inside the mitochondria to release energy. In biological systems, oxidation of hydrogen is coupled with the synthesis of adenosine triphosphate (ATP). ATP is the readily available form of energy (“molecular energy currency unit”) in the cell. ATP has three components: a nitrogenous base (adenine), the sugar ribose, and the triphosphate. Energy is stored within the PO4 bonds, and the release of each phosphate bond generates eight kcal of energy.
ATP is the compound used as an energy source in biochemical reactions
The fate of absorbed glucose and metabolic conversions in the animal body
Absorbed glucose could be utilised by the animal for several functions. The first priority is the formation of glycogen in the liver, which is stored in muscle and hepatic tissue. However, the body stores very little as glycogen (a starch-like compound), and glycogen could be rapidly hydrolysed back to glucose through a process called glycogenolysis. This helps in maintaining blood glucose at a narrow range in normal healthy animals. The second priority is oxidation to form energy (a major function) and is described in the next sections. Since glycogen storage is limited, excess glucose is converted to fat and is stored in adipose tissue. This is accomplished by the breakdown of glucose to pyruvate through a process called glycolysis, which is then available for fat synthesis.
- Storage as glycogen in liver and muscle (small amount)
- Oxidization for energy
- Fat synthesis and storage as fat in adipose tissue
How cells derive energy from glucose: Metabolic pathways
Glucose oxidation is the central reaction of energy metabolism in a cell. Almost all energy that is being generated for a cell to function is by glucose oxidation.
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + energy
Oxidation of glucose is not a one step process.
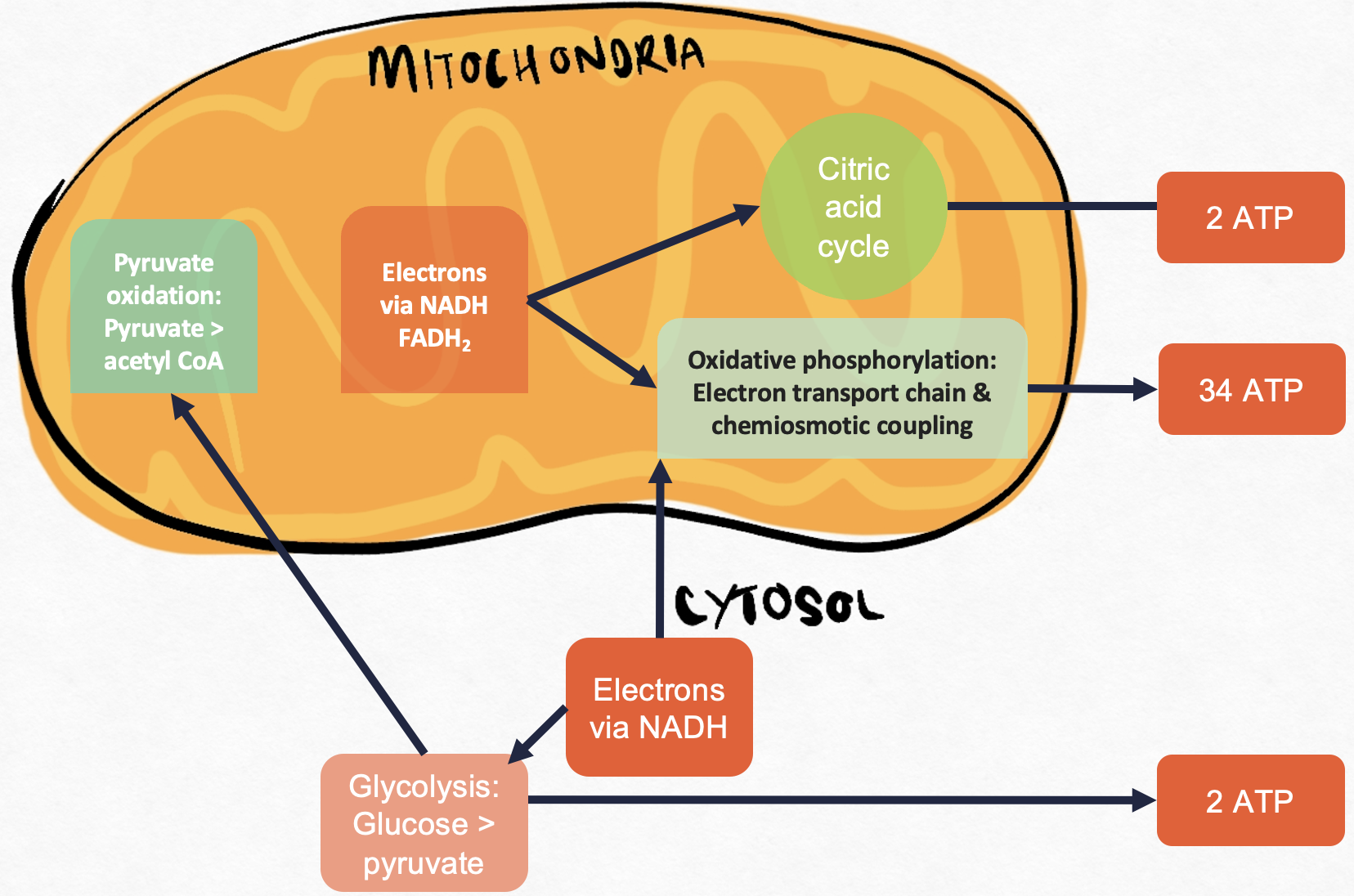
Performed in three stages and occurs in different parts of a cell (Figure 5.8):
1) Glycolysis (in the cytosol)
2) Krebs cycle / citric acid cycle / TCA cycle (in mitochondrial matrix)
3) Oxidative phosphorylation (in the inner mitochondrial membrane)
 Reflective question
Reflective question
How many ATP molecules generated from a single oxidative phosphorylation?
Example
Calculations for one glucose molecule:
- 2 NADH from one glycolysis
- 2 NADH from linking step ( 2 pyruvate → 2 Acetyl CoA)
- 6 NADH and 2 FADH2 from 2 Krebs cycles
- Total = 10 NADH molecules + 2 FADH2 molecules
Approximately 2.5 ATP molecules are made from one NADH
Approximately 1.5 ATP molecules are made from one FADH2
For a single oxidative phosphorylation: 10 NADH X 2.5 ATP/NADH + 2 FADH2 X 1.5 ATP/ FADH2 = 28 ATP
Therefore, ATP molecules generated from a single oxidative phosphorylation is 28
Glycolysis
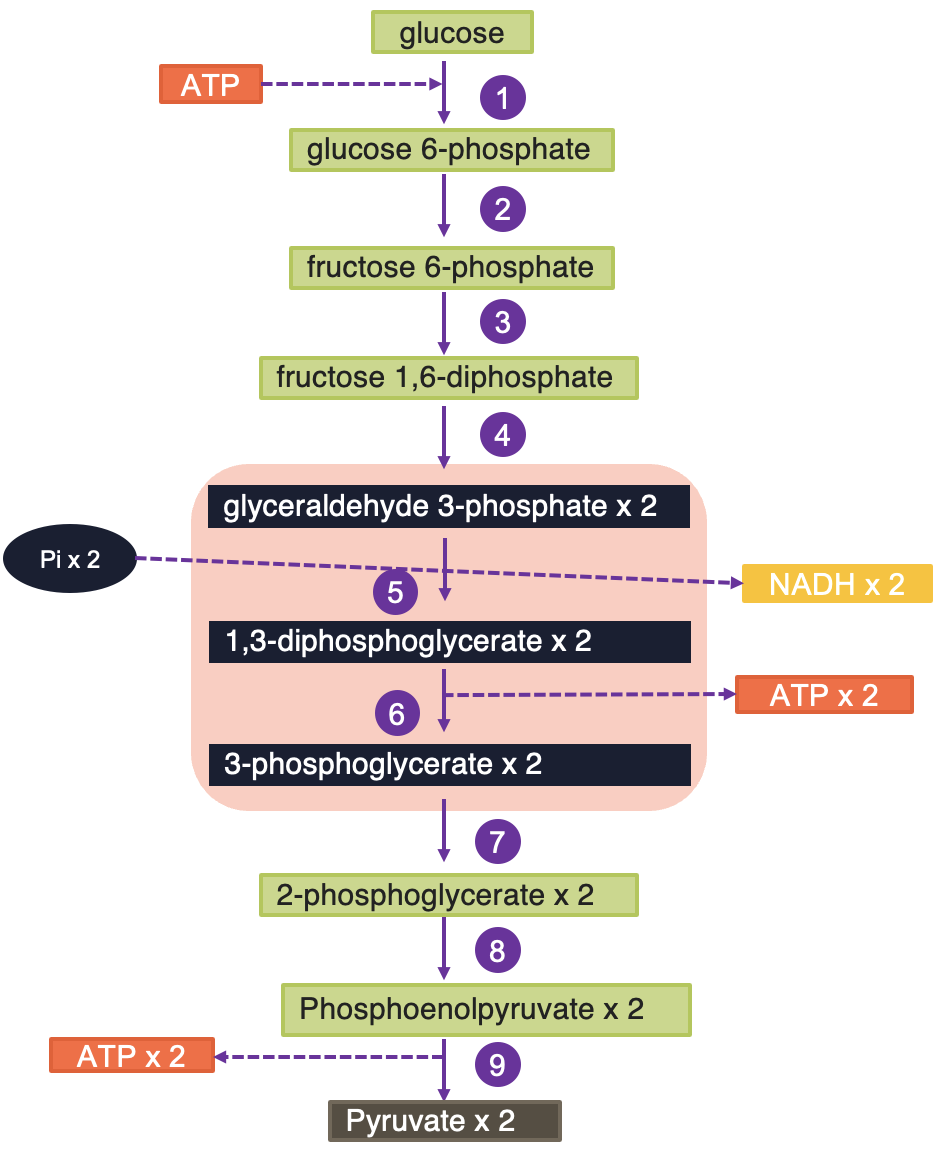
Enzymes for glycolysis are located in the cytosol of the cell, and glycolysis occurs in this part of the cell (Figure 5.9). Glycolysis is the breakdown of 6 C glucose into two 3 C end product pyruvates in aerobic metabolism and lactic acid in anaerobic metabolism. It is a catabolic pathway involving oxidation and yields ATP and NADH (reduced NAD) energy. Glycolysis is the pathway by which other sugars (e.g., fructose, galactose) are catabolised by converting them to intermediates of glycolysis. Fructose can be converted to fructose-6-phosphate by hexokinase. Galactose can enter glycolysis by being converted to galactose-1-phosphate followed by conversion (ultimately) to glucose-1-phosphate and subsequently to glucose-6-phosphate (G6P), which is a glycolysis intermediate.
Energy production process through glycolysis: Glycolysis has two phases: an energy investment phase requiring the input of ATP (preparatory phase) and an energy realisation phase (pay off) where ATP is made. Cells that utilise glucose have an enzyme called hexokinases, which use ATP to phosphorylate the glucose (attaches a phosphorus group) and changes it into G6P. At this point, the cellular “machinery” can begin to process the glucose. Briefly, in the first reaction of glycolysis, hexokinase catalyses the transfer of phosphate to glucose from ATP, forming glucose-6-phosphate. Thus this step uses ATP, which provides the energy necessary for the reaction to proceed. Glucose-6-phosphate is converted to fructose-6-phosphate and subsequently to fructose-1,6-biphosphate, which is cleaved to dihydroxy acetone phosphate (DHAP) and glyceraldehyde-3-phosphate (G3P). During this process, an additional ATP is required to phosphorylate the intermediate fructose-6-phosphate. Therefore, the “preparation” of glucose results in two molecules of ATP being used for every glucose molecule processed.
During the payoff phase, G3P is further processed to produce pyruvate. During this phase, one NADH and two ATP are produced during the intermediate steps. The DHAP produced can be simply converted into G3P and processed in a similar manner as the first G3P. Therefore, one glucose molecule will result in the production of two NADH, four ATP, and two pyruvate molecules.
 Reflective question
Reflective question
What is the net gain of ATP molecules generated by glycolysis?
Example
Calculations for glycolysis (Aerobic)
Input: = 1 glucose molecule
Requires = − 2 ATP (Activation)
Produces:
+ 2 pyruvate molecules
+ 4 ATP
+ 2 NADH (= 6 ATP)
Total = 6 + 4 =1 0 ATP
Net gain =10 − 2 = 8 ATP
Glycolysis: Overall functions
ATP production
For each molecule of glucose, 2 ATP (preparatory phase) were used and 2 NADH, 4 ATP, and 2 pyruvate molecules (payoff phase) were generated; which equals a net production of 2 NADH, 2 ATP, and 2 pyruvate molecules, and the net gain of ATP is 8 per mole of glucose.
Production of other intermediates
Glycolysis provides pyruvate for the citric acid cycle, amino acid synthesis through transamination, glucose-6-phosphate (glycogen synthesis), nicotinamide adenine dinucleotide phosphate, (NADPH) (fatty acid synthesis; triglyceride synthesis), and dihydroxyacetone phosphate for glycerol synthesis (the backbone of fat).
Dive deeper
Glycolysis is a complex process. Watch this short video on ATP and respiration: CrashCourse. (2012, March 13). ATP & respiration: Crash course biology #7 [YouTube, 13:25mins]
 Case study
Case study
A 5-year-old Cocker Spaniel presents to the clinic with symptoms of severe anemia, exercise intolerance, and muscle cramping. The owner reports that the dog becomes easily fatigued during walks and exhibits noticeable weakness after minimal exertion.Upon physical examination, the dog shows signs of pallor and lethargy. Blood tests reveal a significant reduction in red blood cell count and hemoglobin levels. Further biochemical analysis indicates a deficiency in the phosphofructokinase (PFK) enzyme activity.Based on the clinical presentation and laboratory findings, the diagnosis of Phosphofructokinase Deficiency (PKFD) is confirmed. This autosomal recessive disorder, primarily seen in Cocker Spaniels, results from the malfunction of the PFK enzyme in the glycolysis pathway, critically affecting red blood cells that rely solely on glycolysis for energy production.

Fates of pyruvate in the animal body
It is important to discuss the fate of pyruvate generated through glycolysis. Pyruvate has different fates, depending on the conditions of the animal and the cell type.
Fates of pyruvate:
- Lactic acid production
- Acetyl CoA production
Lactic acid production
When oxygen is present, there is plenty of NAD+, so aerobic cells convert pyruvate to acetyl coenzyme A (CoA) for oxidation in the citric acid cycle. When oxygen is absent, NAD+ levels can go down, so to prevent that from happening, lactate dehydrogenase uses NADH and pyruvate is converted to either lactate (animals) or ethanol (bacteria/yeast). Anaerobic conversion of NADH to NAD+ provides much less ATP energy to cells than when oxygen is present. Anaerobic metabolism of glucose generates only two ATP per glucose. Once oxygen is depleted for the cell, another system will convert the lactic acid back to pyruvate and produce glucose.
Anaerobic metabolism of glucose produces two ATP per glucose molecule.
Acetyl CoA production
Acetyl CoA Production: Acetyl CoA production occurs in the aerobic state and serves as the main precursor for the citric acid cycle, lipogenesis, and ketogenesis (during negative balance). Acetyl CoA is converted to ATP through different steps in the citric acid cycle. During this conversion, the enzyme pyruvate dehydrogenase and different B vitamin–containing coenzymes (thiamine, riboflavin, niacin, pantothenic acid) function through a series of condensation, isomerization, and dehydrogenation reactions and produces several different intermediates that are used for fat or amino acid synthesis.
Pyruvate dehydrogenase links glycolysis with the citric acid cycle by converting pyruvate into acetyl CoA.
To generate more energy from the glucose molecule, further biochemical processes occur within the animal body. These include the enzymatic step pyruvate dehydrogenase (PDH), which connects glycolysis (cytosol) with the citric acid cycle in the mitochondria. During this step, 3 C pyruvate is converted to an active form of acetic acid called acetyl CoA, and CO2 is produced.
Pyruvic acid is decarboxylated and the 2 H ions are picked up by NAD+ and thus it provides two mole of NADH for each mole of glucose (net = 6 ATP produced). This enzymatic step needs coenzyme A and its activity is highly regulated by the concentration of acetyl CoA, ATP, and NADH.
Through PDH, one mole of glucose produces 2 NADH or 6 ATP.
The citric acid cycle (also known as the TCA or Kreb’s Cycle)
TCA cycle, citric acid cycle, or Kreb’s cycle (named after Hans Krebs who discovered the pathway in 1937) includes a series of enzyme-catalysed chemical reactions that occur in the matrix of the mitochondria. Several B vitamin–containing enzymes function as coenzymes in the pathway (e.g., thiamine, riboflavin, niacin). The citric acid cycle is the key part of aerobic respiration in cells. The citric acid cycle also serves as a source of precursors for storage forms of fuels (lipids) and building blocks, such as amino acids, in the animal body.
Functions of the citric acid cycle
- Recover more chemical energy
- Provide metabolic intermediates (e.g., citrate, α-ketoglutarate, oxaloacetate)
The citric acid cycle is the central metabolic hub of the cell and occurs in the mitochondria.
Prior to entrance into the cycle, pyruvic acid is converted to acetyl CoA through PDH as described previously. Acetyl CoA (2 C) enters the cycle by combining with a 4 C compound called oxaloacetate and forms a 6 C citric acid. This reaction “pulls” the cycle forward. Citric acid undergoes a series (about 10) of enzyme-catalysed conversions producing different intermediates (e.g., α-ketoglutarate, succinate, fumarate, malate; shown in Figure 5.10).
In many of these steps, high-energy electrons are released to NAD, and NAD molecules also acquire H+ and become NADH. In one of the steps, FAD serves as the electron acceptor, and it acquires H+ and becomes FADH2. In one of the reactions, one ATP is also synthesised from one acetyl CoA. At the end of the cycle, the final product is oxaloacetic acid, which is identical to the oxaloacetic acid that begins the cycle and will pick up another acetyl CoA to begin another turn of the cycle. Altogether, the citric acid cycle produces (per one mole of glucose or two moles of pyruvic acid), two ATP molecules, six NADH, and two FADH2. Both NADH and FADH are used in the electron transport chain to generate ATP. Overall, a net gain of 24 ATP per mole of glucose is obtained through the citric acid cycle.
One mole of glucose produces 24 ATP through the citric acid cycle.
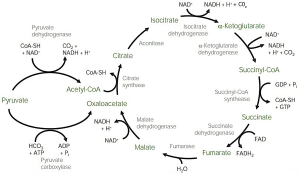
Figure 5.10. The citric acid cycle in the mitochondria. This shows the intermediates of the process. Image by Lecturio. CC BY-NC-SA 4.0.
The citric acid cycle is the final common pathway for oxidation of carbohydrates, fats, and proteins that provides energy.
Overall, for every glucose molecule fully metabolised to CO2 and H2O, we receive 38 ATP. There are eight kcal of energy in every ATP high-energy phosphate bond. Hence net recovery of energy is 38 × 8 = 304 kcal. The efficiency of converting glucose bond energy into ATP high-energy P bond is therefore 304/674* × 100 = 45%.
Altogether, one mole of glucose produces the equivalent of 38 ATP (8 + 6 + 24), or 304 kcal, through the different steps explained.
The rate-limiting step in the citric acid cycle is the combination of oxaloacetate and acetyl CoA to produce citrate. Because of this, it is essential to have adequate quantities of oxaloacetate in order to produce ATP and maintain cell viability. Nutritional diseases such as ketosis result from a deficiency in energy production and therefore require oxaloacetate as precursors to “jump-start” the citric acid cycle. The citric acid cycle also produces intermediates that serve as a precursor for the synthesis of fatty acids and amino acids. For example, citrate can be used for fatty acid synthesis, while oxaloacetate can be used for nonessential amino acids (glucogenic amino acids or most of the nonessential amino acids, e.g., alanine, serine, cysteine, glycine). During low glucose conditions, these amino acids can produce glucose and α-ketoglutarate can be used for glutamic acid synthesis.
Citric acid cycle intermediates: Roles
- Citrate for fatty acid synthesis
- α-ketoglutarate for glutamic acid (amino acid) synthesis
- Oxaloacetate for non essential amino acid synthesis
Electron transport system, respiratory chain, or oxidative phosphorylation
The electron transport chain permits recovery of redox energy associated with NADH and FADH. H ions react with O2 and form water, and thus the electron transport system serves as a source of metabolic water. Electrons are carried to the electron transport system in the mitochondrial wall by NADH and FADH2. Coenzyme Q accepts a pair of electrons and passes electrons singly to cytochrome C and acts as a “traffic cop” for electrons. Oxygen is the terminal electron acceptor and if oxygen is not available, electrons will not pass through the electron transport system and NADH and FADH2 will not be reoxidized. For these reasons, the citric acid cycle will not run either. This is part of metabolic control. Interruption of electron flow can result in production of reactive oxygen species (free radicals). Cellular enzymes, such as superoxide dismutase and catalase, help deactivate reactive oxygen species.
Ruminant carbohydrate metabolism
Volatile fatty acids (VFAs) are produced in large amounts through ruminal fermentation and are of great importance in that they provide greater than 70% of the ruminant’s energy supply. The rumen epithelium performs efficient absorption of VFAs through diffusion through a concentration gradient. As they pass through the epithelium, the different VFAs undergo different degrees of metabolism.
The fate of volatile fatty acids
- Acetate—precursor to Acetyl CoA (citric acid cycle; fat synthesis)
- Propionate—precursor to glucose (glycolysis; milk lactose)
- Butyrate—precursor to Acetyl CoA (citric acid cycle)
Acetate and propionate pass through the epithelium largely unchanged, but almost all the butyric acid is metabolized in the epithelium to beta-hydroxybutyric acid, a type of ketone body.
The three major VFAs (acetic, propionic, butyric) absorbed from the rumen have somewhat distinctive metabolic fates:
Acetic acid is utilised minimally in the liver and is oxidised throughout most of the body to generate ATP. Another important use of acetate is as the major source of acetyl CoA, and it enters the citric acid cycle. Acetic acid is used for the synthesis of lipids (e.g., milk or body fat). A high-roughage diet favours the production of acetic acid.
Propionic acid is almost completely removed from portal blood by the liver. Propionate is converted to succinyl CoA, and it enters the citric acid cycle. Within the liver, propionate serves as a major substrate for gluconeogenesis, which is absolutely critical to the ruminant because almost no glucose reaches the small intestine for absorption. For example, in a dairy cow, all the glucose in the milk lactose was synthesised in the liver and most of that synthesis was from propionic acid. A high-concentrate diet favours the production of propionic acid.
For butyric acid, butyrate is split into two acetyl CoAs, and it enters the citric acid cycle. Most of the butyric acid that comes out of the rumen as the ketone beta-hydroxybutyric acid which oxidised in many tissues for energy production.
Gluconeogenesis
In most cells, glucose-6-phosphate enters the glycolytic pathway and forms pyruvate. In kidney and liver cells glucose-6-phosphatase converts glucose-6-phosphate to glucose. Gluconeogenesis is the process of formation of new glucose molecules and consumes ATP. (It is essentially the reverse of glycolysis).
Glucose can be made from three sources:
1) glycerol – product of break down of triglycerides
- Glycerol is converted to glycerol phosphate – dihydroxyacetone phosphate – glucose
2) lactate – waste product of cells transported to liver for gluconeogenesis
- Lactate is converted to pyruvate – glucose
3) amino acids – product of the breakdown of proteins
- Amino acids – keto acids – pyruvate – glucose
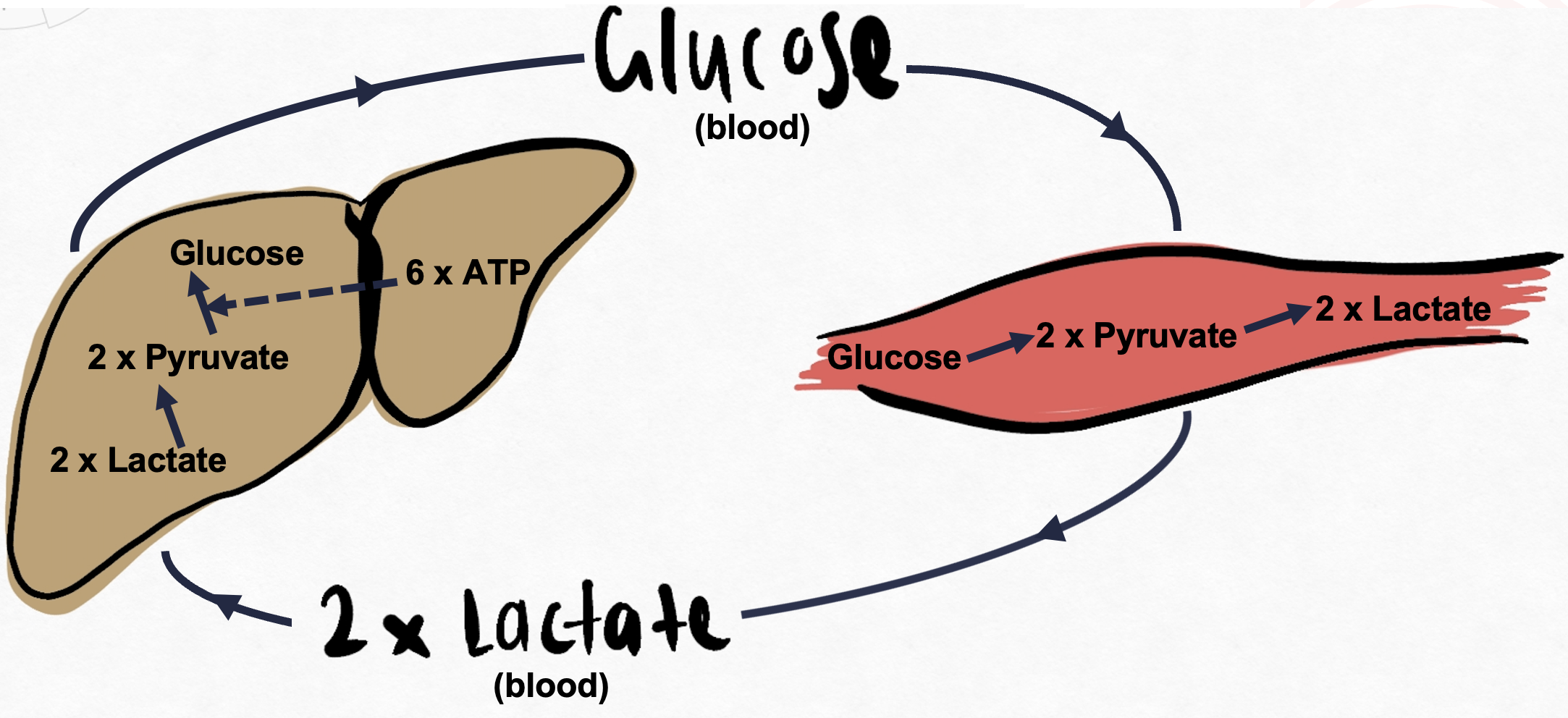
The lactic acid cycle (also known as the Cori Cycle) is the gluconeogenesis pathway from skeletal muscle lactate (Figure 5.11). It is a metabolic pathway in which lactate created by anaerobic glycolysis in muscles is transported to the liver and converted to glucose, which is then metabolised back to lactase in the muscles. Lactate is transferred via blood to the liver and is converted to pyruvate to finally produce glucose. Return of newly synthesised glucose to muscle. Note: Glucose 6 phosphatase enzyme is absent in skeletal muscle.
Knowledge check

