24 Enzymes
It is important to know that the chemical reactions of metabolic pathways do not take place on their own. A substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyse biochemical reactions are proteins called enzymes. Enzymes are important for catalysing all types of biological reactions—those that require energy as well as those that release energy.
All enzymes are proteins but not all proteins are enzymes
Most enzymes perform the critical task of lowering the activation energies of chemical reactions inside the cell (Figure 5.2). Most of the reactions critical to a living cell happen too slowly at normal temperatures to be of any use to the cell. Without enzymes to speed up these reactions, life could not persist. Enzymes do this by binding to the reactant molecules and holding them in such a way as to make the chemical bond-breaking and -forming processes take place more easily. It is important to remember that enzymes do not change whether a reaction is exergonic (spontaneous) or endergonic. This is because they do not change the free energy of the reactants or products. They only reduce the activation energy required for the reaction to go forward. In addition, an enzyme itself is unchanged by the reaction it catalyses. Once one reaction has been catalysed, the enzyme is able to participate in other reactions.
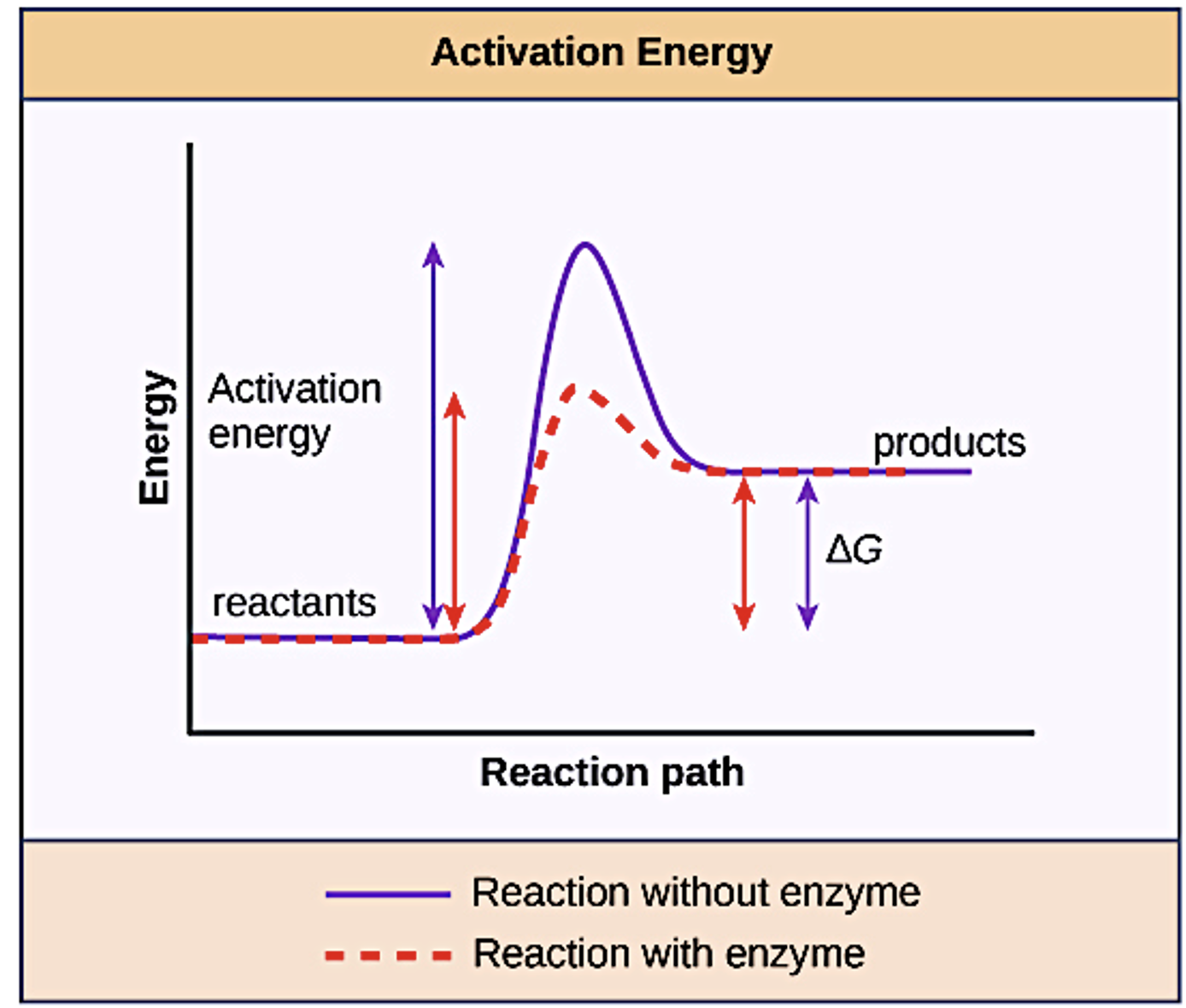
Enzymes are specialised catalysts that increase the rate of a chemical reaction.
An enzyme binds to a reactant called a substrate, forming an intermediate enzyme-substrate complex in a reversible reaction. The substrate interacts with the enzyme through weak bonds but may dissociate before the reaction proceeds. If the substrate remains bound to the enzyme long enough, a product is formed. Importantly, the enzyme remains unchanged after the reaction and can act on a new substrate molecule to generate another product.
Enzymes usually catalyse one specific reaction and are able to recognise and bind to only one type of substrate, which is known as substrate specificity. Substrate specificity depends on the complementary shapes of the enzyme and substrate molecule. In other words, how closely does a substrate molecule fit and bind to a particular site (active site) on the enzyme molecule.
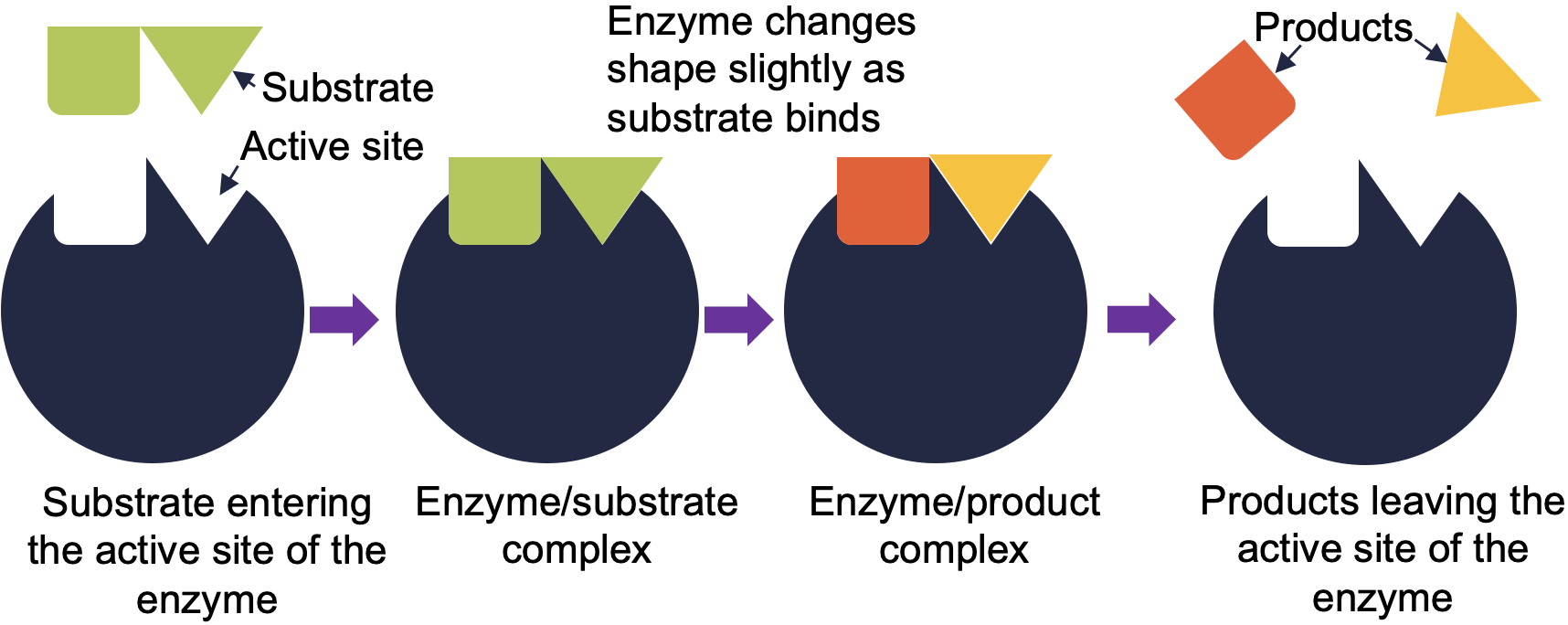
For many years, scientists thought that enzyme-substrate binding took place in a simple ‘lock and key’ fashion. This model asserted that the enzyme and substrate fit together perfectly in one instantaneous step. However, current research supports a model called induced fit (Figure 5.3). The induced-fit model expands on the lock-and-key model by describing a more dynamic binding between enzyme and substrate. As the enzyme and substrate come together, their interaction causes a mild shift in the enzyme’s structure that forms an ideal binding arrangement between enzyme and substrate.
When an enzyme binds its substrate, an enzyme-substrate complex is formed. This complex lowers the activation energy of the reaction and promotes its rapid progression in one of the multiple possible ways. On a basic level, enzymes promote chemical reactions that involve more than one substrate by bringing the substrates together in an optimal orientation for reaction. Another way in which enzymes promote the reaction of their substrates is by creating an optimal environment within the active site for the reaction to occur. The chemical properties that emerge from the particular arrangement of amino acid R groups within an active site create the perfect environment for an enzyme’s specific substrates to react.
The enzyme-substrate complex can also lower activation energy by compromising the bond structure so that it is easier to break. Finally, enzymes can also lower activation energies by taking part in the chemical reaction itself. In these cases, it is important to remember that the enzyme will always return to its original state upon the completion of the reaction. One of the hallmark properties of enzymes is that they remain ultimately unchanged by the reactions they catalyse. After an enzyme has catalysed a reaction, it releases its product(s) and can catalyse a new reaction.
Two theories explaining the substrate specificity:
1 ) Lock and Key Model
- A limitation of this model is that it does not explain how these enzyme substrate reactions are reversible.
2) Induced-fit model
- This model more likely explains reversible reactions that are enzyme catalysed
- In this model, the shapes of the substrate and active site of the enzyme are similar but not complementary as seen in lock and key model
- When substrate binds to the enzyme causes a conformational change in the enzyme a better fit of the substrate in the active site of enzyme
Dive deeper
Watch Quick Biochemistry Basics. (2020, January 4). Induced fit model [YouTube, 1:52mins]
Since the rates of biochemical reactions are controlled by activation energy, and enzymes lower and determine activation energies for chemical reactions, the relative amounts and functioning of the variety of enzymes within a cell ultimately determine which reactions will proceed and at what rates. This determination is tightly controlled in cells. In certain cellular environments, enzyme activity is partly controlled by environmental factors like pH, temperature, salt concentration, and, in some cases, cofactors or coenzymes.
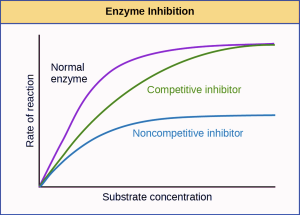
Enzymes can also be regulated in ways that either promote or reduce enzyme activity. There are many kinds of molecules that inhibit or promote enzyme function, and various mechanisms by which they do so. In some cases of enzyme inhibition, an inhibitor molecule is similar enough to a substrate that it can bind to the active site and simply block the substrate from binding. When this happens, the enzyme is inhibited through competitive inhibition, because an inhibitor molecule competes with the substrate for binding to the active site.
On the other hand, in noncompetitive inhibition, an inhibitor molecule binds to the enzyme in a location other than the active site, called an allosteric site, but still manages to block substrate binding to the active site. Some inhibitor molecules bind to enzymes in a location where their binding induces a conformational change that reduces the affinity of the enzyme for its substrate. This type of inhibition is called allosteric inhibition. Most allosterically regulated enzymes are made up of more than one polypeptide, meaning that they have more than one protein subunit. When an allosteric inhibitor binds to a region on an enzyme, all active sites on the protein subunits are changed slightly such that they bind their substrates with less efficiency.
Enzymes are key components of metabolic pathways. Understanding how enzymes work and how they can be regulated are key principles behind the development of many of the pharmaceutical drugs on the market today.
 Case study
Case study
A 7-year-old Schnauzer with a enzyme HMG-CoA reductase deficiency requires yearly check ups to monitor their increased cholesterol and triglyceride levels, though this is predominantly managed through diet. For dogs who are unresponsive to dietary changes, statins are a class of drugs that can reduce cholesterol levels. Statins, such as simvastatin, are used in medicine to manage conditions like hyperlipidemia. By inhibiting the enzyme HMG-CoA reductase, the level of cholesterol synthesised in the body can be reduced.

How are drugs discovered?
One of the biggest challenges in drug discovery is identifying a drug target. A drug target is a molecule that is literally the target of the drug. In the case of statins, HMG-CoA reductase is the drug target. Drug targets are identified through painstaking research in the laboratory. Identifying the target alone is not enough; scientists also need to know how the target acts inside the cell and which reactions go awry in the case of a disease. Once the target and the pathway are identified, then the actual process of drug design begins. In this stage, chemists and biologists work together to design and synthesise molecules that can block or activate a particular reaction. However, this is only the beginning: If and when a drug prototype is successful in performing its function, then it is subjected to many tests from in vitro experiments to clinical trials before it can get approval from the U.S. Food and Drug Administration to be on the market.
Cofactors and coenzymes
Many enzymes do not work optimally, or even at all, unless bound to other specific non-protein helper molecules. They may bond either temporarily through ionic or hydrogen bonds, or permanently through stronger covalent bonds. Binding to these molecules promotes optimal shape and function of their respective enzymes. Two examples of these types of helper molecules are cofactors and coenzymes. Cofactors are inorganic ions such as ions of iron and magnesium. Coenzymes are organic helper molecules, those with a basic atomic structure made up of carbon and hydrogen. Like enzymes, these molecules participate in reactions without being changed themselves and are ultimately recycled and reused. Vitamins are the source of coenzymes. Some vitamins are the precursors of coenzymes and others act directly as coenzymes. Vitamin C is a direct coenzyme for multiple enzymes that take part in building the important connective tissue, collagen. Therefore, enzyme function is, in part, regulated by the abundance of various cofactors and coenzymes, which may be supplied by an organism’s diet or, in some cases, produced by the organism.
Cofactors are necessary for enzymes to function properly. Examples of cofactors include metal ions such as iron, copper, or zinc. Coenzymes are non-protein organic molecules mostly derived from vitamins.
Coenzymes important for energy metabolism
- Flavin adenine dinucleotide (FAD): A derivative of vitamin B12 (riboflavin)
- Nicotinamide adenine dinucleotide (NAD): A derivative of vitamin B3 (niacin)
- Coenzyme A (CoA): A derivative of vitamin B5 (pantothenic acid)
The rate of enzymatic reactions
The rate of a chemical reaction is a measure of how fast it consumes the reactants and products are formed. The rate of metabolic reaction is important. For a body to function properly, the rate of reactions in the body at par with the needs of the body. If the rate is higher or lower, it can result in cellular damage. Several factors affect the rate of reactions.
Active sites are subject to influences of the local environment. Increasing the environmental temperature generally increases reaction rates, enzyme-catalysed or otherwise. However, temperatures outside of an optimal range reduce the rate at which an enzyme catalyses a reaction. Hot temperatures will eventually cause enzymes to denature, an irreversible change in the three-dimensional shape and therefore the function of the enzyme. Enzymes are also suited to function best within a certain pH and salt concentration range, and, as with temperature, extreme pH, and salt concentrations can cause enzymes to denature.
1. Reactant and product concentration
- If more reactants, then the forward rate of reaction increases
- If more products, then the reverse rate of reaction increases
2. Temperature
- Generally, the rate of a reaction increases with higher temperatures and vice versa.
3. Substrate concentration
- With an in substrate con. , the chances of substrate binding to the enzyme
- After a point all sites of the enzyme are occupied by the substrate (100% saturated)
4. Enzyme concentration
- With an increase in enzyme concentration, the chances of enzyme binding to the substrate are increased
- Rate of the reaction increases with an increase in the concentration of enzyme
5. Catalytic rate of enzyme
- Reflects how fast an enzyme can carry out the catalytic step
- Rate of an enzymatic reaction increases with increase in the catalytic rate of an enzyme
6. Affinity of the enzyme to the substrate (Figure 5.4)
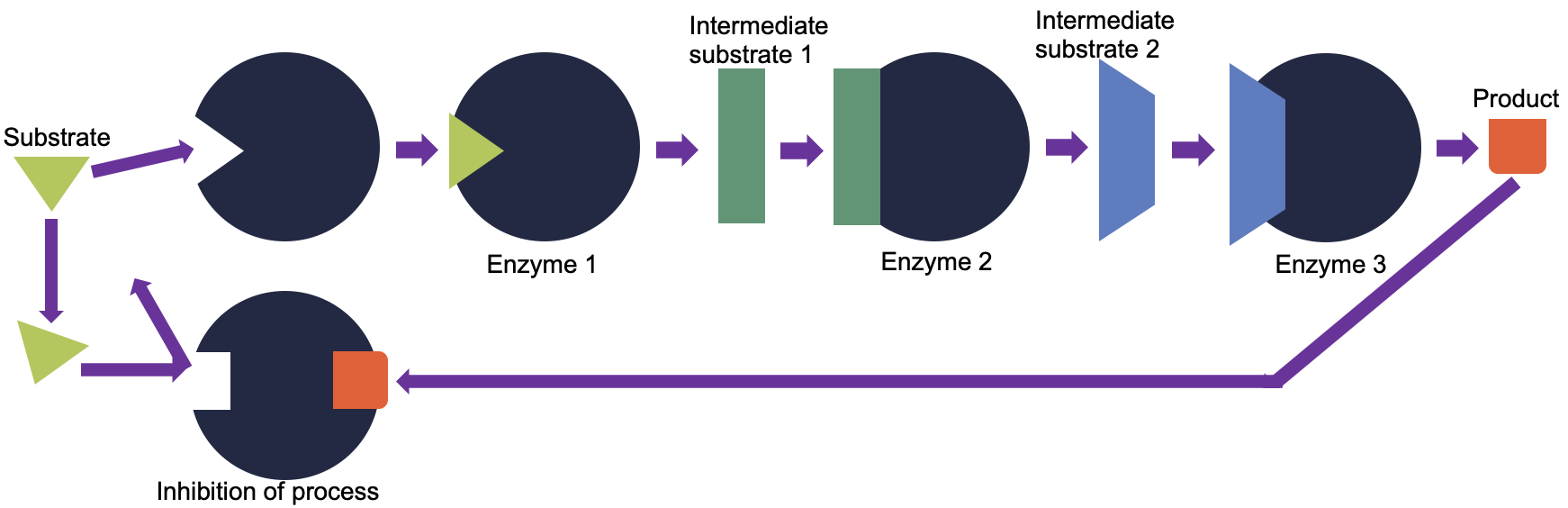
Cells have evolved to use the products of their own reactions for feedback inhibition of enzyme activity. Feedback inhibition involves the use of a reaction product to regulate its own further production (Figure 5.5). The cell responds to an abundance of the products by slowing down production during anabolic or catabolic reactions.
Example
The production of both amino acids and nucleotides is controlled through feedback inhibition. Additionally, ATP is an allosteric regulator of some of the enzymes involved in the catabolic breakdown of sugar, the process that creates ATP. In this way, when ATP is in abundant supply, the cell can prevent the production of ATP. On the other hand, ADP serves as a positive allosteric regulator (an allosteric activator) for some of the same enzymes that are inhibited by ATP. Thus, when relative levels of ADP are high compared to ATP, the cell is triggered to produce more ATP through sugar catabolism.

