33 Active transport
On this page
- Carrier proteins for active transport
- Primary active transport
- Secondary active transport (co-transport)
- Bulk transport
- Calcium signalling
To move substances against a concentration or electrochemical gradient, the cell must use energy. This energy comes from ATP generated through the cell’s metabolism. Active transport mechanisms, or pumps, work against electrochemical gradients. Small substances constantly pass through plasma membranes. Active transport maintains concentrations of ions and other substances that living cells require in the face of these passive movements. A cell may spend much of its metabolic energy supply maintaining these processes. (A red blood cell uses most of its metabolic energy to maintain the imbalance between exterior and interior sodium and potassium levels that the cell requires.) Because active transport mechanisms depend on a cell’s metabolism for energy, they are sensitive to many metabolic poisons that interfere with the ATP supply.
Two mechanisms exist for transporting small-molecular weight material and small molecules. Primary active transport moves ions across a membrane and creates a difference in charge across that membrane, which is directly dependent on ATP. Secondary active transport does not directly require ATP: instead, it is the movement of material due to the electrochemical gradient established by primary active transport
Carrier proteins for active transport
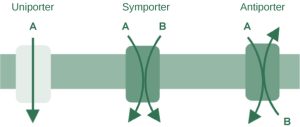
An important membrane adaptation for active transport is the presence of specific carrier proteins or pumps to facilitate movement: there are three protein types or transporters (Figure 6.7). A uniporter carries one specific ion or molecule. A symporter carries two different ions or molecules, both in the same direction. Conversely, antiporters are secondary active transport systems that transport substances in opposite directions. For example, the sodium-hydrogen ion antiporter uses the energy from the inward flood of sodium ions to move hydrogen ions (H+) out of the cell. The sodium-hydrogen antiporter is used to maintain the pH of the cell’s interior. These three types of carrier proteins are also in facilitated diffusion, but they do not require ATP to work in that process. Some examples of pumps for active transport are Na+-K+ ATPase, which carries sodium and potassium ions, and H+-K+ ATPase, which carries hydrogen and potassium ions. Both of these are antiporter carrier proteins. Two other carrier proteins are Ca2+ ATPase and H+ ATPase, which carry only calcium and only hydrogen ions, respectively. Both are pumps.
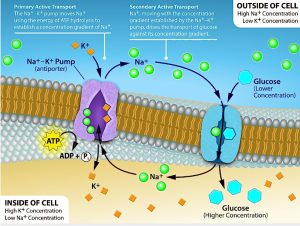
Primary active transport
The primary active transport that functions with the active transport of sodium and potassium allows secondary active transport to occur.
One of the most important pumps in animal cells is the sodium-potassium pump (Na+-K+ ATPase), which maintains the electrochemical gradient (and the correct concentrations of Na+ and K+) in living cells. The sodium-potassium pump moves K+ into the cell while moving Na+ out at the same time, at a ratio of three Na+ for every two K+ ions moved in. The Na+-K+ ATPase exists in two forms, depending on its orientation to the cell’s interior or exterior and its affinity for either sodium or potassium ions.
The process consists of the following six steps.:
- With the enzyme oriented towards the cell’s interior, the carrier has a high affinity for sodium ions. Three ions bind to the protein.
- The protein carrier hydrolyses ATP and a low-energy phosphate group attaches to it.
- As a result, the carrier changes shape and reorients itself towards the membrane’s exterior. The protein’s affinity for sodium decreases and the three sodium ions leave the carrier.
- The shape change increases the carrier’s affinity for potassium ions, and two such ions attach to the protein. Subsequently, the low-energy phosphate group detaches from the carrier.
- With the phosphate group removed and potassium ions attached, the carrier protein repositions itself towards the cell’s interior.
- The carrier protein, in its new configuration, has a decreased affinity for potassium, and the two ions moves into the cytoplasm. The protein now has a higher affinity for sodium ions, and the process starts again.
Several things have happened as a result of this process. At this point, there are more sodium ions outside the cell than inside and more potassium ions inside than out. For every three sodium ions that move out, two potassium ions move in. This results in the interior being slightly more negative relative to the exterior. This difference in charge is important in creating the conditions necessary for the secondary process. The sodium-potassium pump is, therefore, an electrogenic pump (a pump that creates a charge imbalance), creating an electrical imbalance across the membrane and contributing to the membrane potential.
Dive deeper
Watch this video explaining the sodium potassium pump. Amoeba Sisters (2020, January 30). Sodium Potassium Pump [YouTube, 7:00mins]
 Case study
Case study
Whiskers, a 6-year-old male domestic shorthair cat, presented with symptoms of lethargy, weakness, rapid breathing, and muscle tremors. His owner reported that these symptoms had developed over the past two days, and additionally, Whiskers’ urine appeared dark in colour. During the physical examination, Whiskers exhibited pale mucous membranes, an elevated heart rate, and respiratory rate, along with noticeable muscle tremors. Blood tests revealed a low red blood cell (RBC) count and elevated reticulocyte count, indicating regenerative anaemia. Serum potassium levels were elevated, confirming hyperkalaemia. A blood smear showed signs of haemolysis, and urinalysis confirmed the presence of haemoglobin in the urine, indicating haemolysis.
Based on the clinical signs and diagnostic tests, Whiskers was diagnosed with hyperkalaemia due to kidney failure. Hyperkalemia is characterised by elevated potassium levels in the blood, which can result from the failure of the sodium-potassium pump. This condition is often seen in animals with kidney failure or urinary tract obstruction, where the kidneys cannot excrete potassium effectively.
Hyperkalaemia is a serious condition that disrupts the normal electrical activity of cells, particularly in the heart and muscles. The failure of the sodium-potassium pump leads to an accumulation of potassium in the blood, causing symptoms such as muscle weakness, cardiac arrhythmias, and in severe cases, cardiac arrest. Common triggers for hyperkalaemia include kidney failure, urinary tract obstruction, certain medications, and Addison’s disease.
Immediate actions include intravenous fluid therapy to restore normal electrolyte levels and promote kidney function. Medications such as calcium gluconate can stabilise the heart’s electrical activity, while insulin and glucose helped shift potassium back into cells. Diuretics were used to promote potassium excretion. Long-term management involved regular monitoring of blood potassium levels and kidney function, dietary modifications to reduce potassium intake, and managing underlying conditions such as kidney disease.

Secondary active transport (co-transport)
Secondary active transport uses the kinetic energy of the sodium ions to bring other compounds, against their concentration gradient into the cell.
As sodium ion concentrations build outside of the plasma membrane because of the primary active transport process, this creates an electrochemical gradient. If a channel protein exists and is open, the sodium ions will move down its concentration gradient across the membrane. This movement transports other substances that must be attached to the same transport protein in order for the sodium ions to move across the membrane (Figure 6.8). Many amino acids, as well as glucose, enter a cell this way. This secondary process also stores high-energy hydrogen ions in the mitochondria of plant and animal cells in order to produce ATP. The potential energy that accumulates in the stored hydrogen ions translates into kinetic energy as the ions surge through the channel protein ATP synthase, and that energy then converts ADP into ATP.
 Reflective question
Reflective question
If the pH outside the cell decreases, would you expect the amount of amino acids transported into the cell to increase or decrease?
Bulk transport
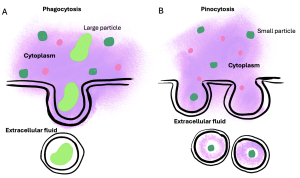
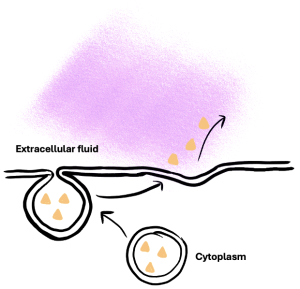
Other forms of active transport do not involve membrane carriers. Endocytosis (bringing ‘into the cell’) is the process of a cell ingesting material by enveloping it in a portion of its cell membrane, and then pinching off that portion of membrane (Figure 6.9). Once pinched off, the portion of membrane and its contents becomes an independent, intracellular vesicle. A vesicle is a membranous sac—a spherical and hollow organelle bounded by a lipid bilayer membrane. Endocytosis often brings materials into the cell that must be broken down or digested. Phagocytosis (‘cell eating’) is the endocytosis of large particles. Many immune cells engage in phagocytosis of invading pathogens. Like little Pac-men, their job is to patrol body tissues for unwanted matter, such as invading bacterial cells, phagocytose them, and digest them. In contrast to phagocytosis, pinocytosis (‘cell drinking’) brings fluid containing dissolved substances into a cell through membrane vesicles.
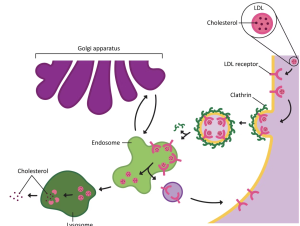
Phagocytosis and pinocytosis take in large portions of extracellular material, and they are typically not highly selective in the substances they bring in. Cells regulate the endocytosis of specific substances via receptor-mediated endocytosis. Receptor-mediated endocytosis is endocytosis by a portion of the cell membrane that contains many receptors that are specific for a certain substance. Once the surface receptors have bound enough of the specific substance (the receptor’s ligand), the cell will endocytose the part of the cell membrane containing the receptor-ligand complexes (figure 6.10). Iron, a required component of haemoglobin, is endocytosed by red blood cells in this way. Iron is bound to a protein called transferrin in the blood. Specific transferrin receptors on red blood cell surfaces bind the iron-transferrin molecules, and the cell endocytoses the receptor-ligand complexes.
In contrast with endocytosis, exocytosis (taking ‘out of the cell’) is the process of a cell exporting material using vesicular transport (Figure 6.11). Many cells manufacture substances that must be secreted, like a factory manufacturing a product for export. These substances are typically packaged into membrane-bound vesicles within the cell. When the vesicle membrane fuses with the cell membrane, the vesicle releases its contents into the interstitial fluid. The vesicle membrane then becomes part of the cell membrane
For example
Cells of the stomach and pancreas produce and secrete digestive enzymes through exocytosis. Endocrine cells produce and secrete hormones that are sent throughout the body, and certain immune cells produce and secrete large amounts of histamine, a chemical important for immune responses.
 Case study
Case study
Cystic fibrosis (CF) is a genetic disease well-known for its damage to the lungs, causing breathing difficulties and chronic lung infections, but it also affects the liver, pancreas, and intestines.
The symptoms of CF result from a malfunctioning membrane ion channel called the cystic fibrosis transmembrane conductance regulator, or CFTR. The CFTR protein is an integral membrane protein that transports Cl– ions out of the cell. In a person who has CF, the gene for the CFTR is mutated, thus, the cell manufactures a defective channel protein that typically is not incorporated into the membrane but is instead degraded by the cell.
The CFTR requires ATP in order to function, making its Cl– transport a form of active transport. This characteristic puzzled researchers for a long time because the Cl– ions are actually flowing down their concentration gradient when transported out of cells. Active transport generally pumps ions against their concentration gradient, but the CFTR presents an exception to this rule.
In normal lung tissue, the movement of Cl– out of the cell maintains a Cl–-rich, negatively charged environment immediately outside of the cell. This is particularly important in the epithelial lining of the respiratory system. Respiratory epithelial cells secrete mucus, which serves to trap dust, bacteria, and other debris. A cilium (plural = cilia) is one of the hair-like appendages found on certain cells. Cilia on the epithelial cells move the mucus and its trapped particles up the airways away from the lungs and toward the outside. In order to be effectively moved upward, the mucus cannot be too viscous; rather it must have a thin, watery consistency. The transport of Cl– and the maintenance of an electronegative environment outside of the cell attract positive ions such as Na+ to the extracellular space. The accumulation of both Cl– and Na+ ions in the extracellular space creates solute-rich mucus, which has a low concentration of water molecules. As a result, through osmosis, water moves from cells and extracellular matrix into the mucus, ‘thinning’ it out. This is how, in a normal respiratory system, the mucus is kept sufficiently watered-down to be propelled out of the respiratory system.
If the CFTR channel is absent, Cl– ions are not transported out of the cell in adequate numbers, thus preventing them from drawing positive ions. The absence of ions in the secreted mucus results in the lack of a normal water concentration gradient. Thus, there is no osmotic pressure pulling water into the mucus. The resulting mucus is thick and sticky, and the ciliated epithelia cannot effectively remove it from the respiratory system. Passageways in the lungs become blocked with mucus, along with the debris it carries. Bacterial infections occur more easily because bacterial cells are not effectively carried away from the lungs.
Dive deeper
Watch: Animated biology with Arpan. (2019, January 24). Calcium Signalling inside the cell [YouTube, 10.18mins]

